

Thursday, Aug 1st 2024 1PM 93°F 4PM 91°F 5-Day Forecast
More than 130 CVS own-brand drugs recalled by FDA - after horrifying truths about how meds are made
By Luke Andrews Senior Health Reporter For Dailymail.ComPublished: 11:20 EDT, 12 June 2024 | Updated: 11:28 EDT, 12 June 20 2024
A new analysis has revealed the dangers of buying CVS' own-brand drugs.
FDA data shows America's largest pharmacy chain recalled 133 over-the-counter medicines over the last decade, or about one a month.
That was more than twice as many as its competitor Walgreens, which had 70 recalls over the same period, and three times more than Walmart, which had 51 recalls.
The reasons for the CVS recalls included drugs being infested with bacteria, mold growing in factory ventilators, peeling paint and barefoot workers in factories and pills containing incorrect doses.
Own-brand eye drops were the CVS products most likely to be recalled over the last decade, followed by own-brand constipation drugs — such as magnesium citrate tablets — and those for treating colds and flus.
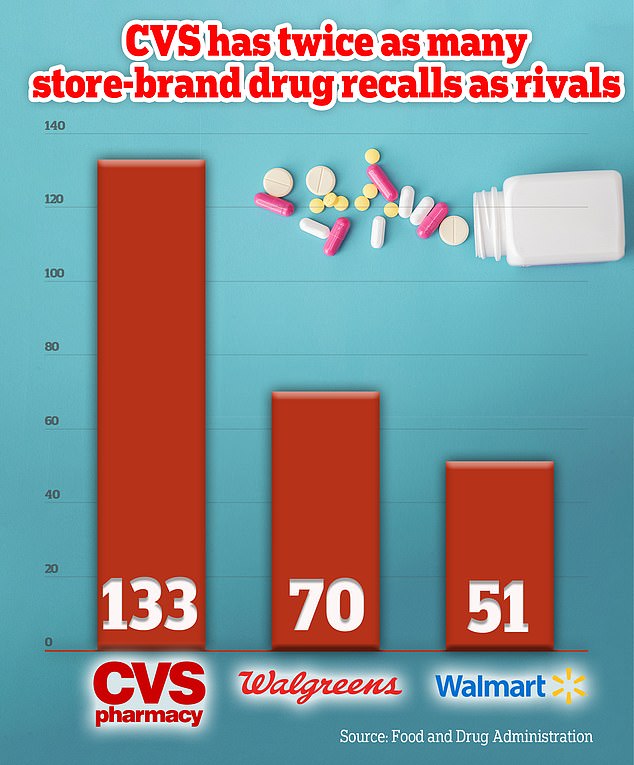
An analysis of recalls showed that over the last decade CVS has recorded 133 recalls, at least twice as many as its two closest rivals

Pictured above is the factory run by Kilitch Healthcare India Limited which made eyedrops sold at stores including CVS, where they also carried the company's label
TRENDING

Sex therapist reveals technique that makes women climax every time
5.7k viewing now

Common pill taken by millions could prevent colon cancer, study finds
3.1k viewing now
People born within three-decade window at higher risk of 17 cancers
2k viewing now
Recalled products were manufactured by companies based in China and India, as well as some in the US — including Tennessee and Florida.
CVS has seen its recalls rise in recent years, recording less than ten a year from 2014 to 2018 but above this number for four out of six years since then.
So far this year, the chain has recorded 11 recalls — mostly for eye drops, cough medicines and drugs for treating constipation.
Experts have been warning for some time over generic medicines, saying there are too few incentives for pharmacy chains to ensure their quality.
This is due to a loophole in FDA rules which makes CVS not responsible for the quality of generics manufactured by third-party factories, even when the products carry a red heart and the words 'CVS Health'.
Dr Kevin Schulman, a medicine expert at Stanford University, told Bloomberg: 'The best way to make a low-price product is to skimp on quality, and that's what we're seeing over and over and over again.'
A spokesperson for CVS said in a statement that the chain — which has more than 9,000 stores nationally — prioritizes 'good manufacturing and ethical sourcing practices'.
They added that CVS brands 'are designed to maximize quality and safety, work as intended, comply with regulations and satisfy customers'.

